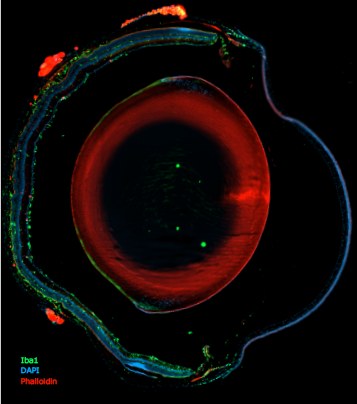
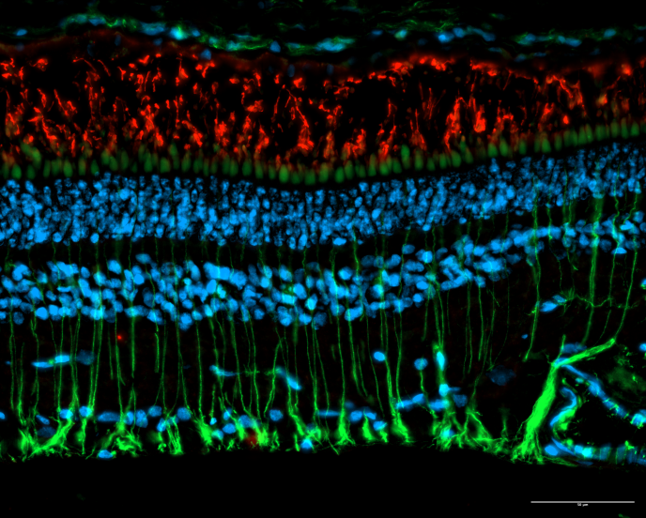
Existem poucos exemplos a nível mundial em que um biólogo celular molecular inicia a sua investigação com a identificação da função de uma proteína e culmina no desenvolvimento de uma intervenção terapêutica disruptiva. O trabalho de Miguel C. Seabra destaca-se pelo seu impacto extraordinário tanto na biologia molecular como na inovação terapêutica, especialmente no campo da terapia genética para doenças raras da retina. Em 1992, Seabra fez uma descoberta revolucionária ao identificar que a proteína REP1 regula as Rab GTPases, essenciais para o transporte intracelular de membranas. Esta descoberta foi diretamente associada à Coroideremia (CHM), uma degeneração retiniana hereditária ligada ao cromossoma X, que leva à cegueira na idade adulta.
Ao longo de mais de 22 anos de investigação intensiva e estudos pré-clínicos, Seabra e a sua equipa lideraram esforços que culminaram num ensaio clínico de fase 1/2 para a terapia genética da CHM, demonstrando tanto a segurança como a eficácia deste tratamento inovador. Este ensaio forneceu uma prova de conceito para uma terapia curativa, revolucionando as perspetivas de tratamento da CHM e de outras doenças retinianas semelhantes, trazendo esperança a pacientes e às suas famílias.
As contribuições de Seabra para a inovação estendem-se também ao empreendedorismo. Em 2014, co-fundou a Nightstar Therapeutics, uma empresa que lançou um ensaio clínico de fase III para a terapia genética da CHM. A empresa foi cotada na NASDAQ em 2017 e adquirida pela Biogen em 2019, consolidando ainda mais o impacto real da investigação desenvolvida por Miguel Seabra.
O trabalho de investigação beneficiou diretamente cerca de 100 pacientes envolvidos nos ensaios clínicos para a terapia genética da CHM, mas o seu trabalho tem implicações mais amplas na evolução dos tratamentos para doenças retinianas em todo o mundo.
De facto, o impacto do trabalho de Miguel Seabra vai muito além desta aplicação específica. O seu trabalho pioneiro em terapia genética abriu caminho para avanços mais amplos no tratamento de doenças raras da retina, demonstrando o potencial das terapias baseadas no Vírus Adeno-Associado (AAV). As suas contribuições não só reformularam as estratégias terapêuticas, como também tiveram um impacto duradouro na compreensão fundamental da biologia celular. A sua investigação sobre as Rab GTPases e o transporte de melanina dos melanócitos para os queratinócitos tornou-se referência, sendo amplamente citada e incluída em manuais científicos.
Reconhecido como um dos cientistas mais citados a nível mundial, Seabra posiciona-se no top 0,2% do ranking da Universidade de Stanford em 2022 e é o cientista biomédico mais bem classificado em Portugal, com um índice h de 74 e cerca de 21.000 citações. O seu trabalho foi publicado em revistas prestigiadas como The Lancet, Nature e Cell, sublinhando ainda mais a sua projeção internacional na ciência.
Para além da investigação e do empreendedorismo, Seabra garantiu mais de 13 milhões de euros em financiamento de organizações prestigiadas, incluindo o Wellcome Trust UK, o MRC UK e a Foundation Fighting Blindness USA. O seu legado inclui a formação e orientação de 23 investigadores de pós-doutoramento e 37 estudantes de doutoramento, muitos dos quais seguiram carreiras de sucesso na academia, na indústria e na formulação de políticas científicas.
Associações de pacientes nos EUA, Canadá, Reino Unido e França, inspiradas pelas descobertas de Seabra, desempenharam um papel crucial no avanço da investigação e na angariação de fundos, acelerando o percurso desde os estudos pré-clínicos até às aplicações clínicas. Embora a terapia ainda não tenha recebido aprovação da FDA ou da EMA, o futuro é promissor para milhares de pacientes que poderão vir a beneficiar destes tratamentos transformadores.
O trabalho de Miguel C. Seabra é um testemunho de como uma descoberta científica fundamental pode evoluir para inovações terapêuticas transformadoras, mudando vidas e o futuro da ciência médica.
Estas conquistas são sempre o resultado de um esforço coletivo, e aproveito esta oportunidade para expressar o meu profundo reconhecimento à equipa dedicada com quem trabalhei ao longo dos anos, bem como à inestimável contribuição dos próprios pacientes, que apoiaram este projeto em momentos críticos. Sinto-me profundamente honrado por ter contribuído para fazer a diferença na vida dos pacientes e para o avanço desta área. Espero que este trabalho inspire jovens cientistas a seguirem as suas paixões e a explorarem novas fronteiras na ciência, pois juntos continuamos a transformar esperança em realidade.
Miguel Seabra